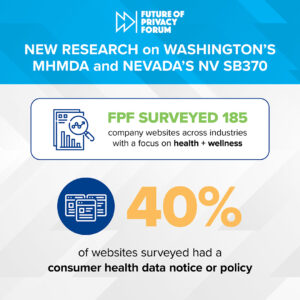
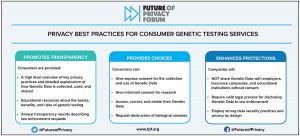
Healthcare technologies are rapidly evolving, producing new data types and innovative data uses. Data and technology can bring significant enhancements to the healthcare system, deepen patients’ and consumers’ engagement and understanding about their health, and be used as part of initiatives meant to improve health outcomes. It is critical to analyze how sensitive health and wellness data affect individual privacy and understand what it means for doctors, researchers, and companies to responsibly use such data. The FPF health team continues to build on its prior work on Consumer Wearables and Wellness Apps and Devices and Privacy Best Practices for Consumer Genetic Testing Services by exploring and addressing issues at the forefront and intersection of health, data, and privacy. Of main focus are the privacy challenges related to the collection, use, and sharing of both medical data and data that falls outside of the scope of HIPAA and FDA regulations. FPF brings together stakeholders to analyze how new technologies and data practices in the health ecosystem can impact individual privacy and promote the more effective and ethical use of data.
Featured
FPF Experts Take The Stage at the 2025 IAPP Global Privacy Summit
By FPF Communications Intern Celeste Valentino Earlier this month, FPF participated at the IAPP’s annual Global Privacy Summit (GPS) at the Convention Center in Washington, D.C. The Summit convened top privacy professionals for a week of expert workshops, engaging panel discussions, and exciting networking opportunities on issues ranging from understanding U.S. state and global privacy […]
FPF Launches Major Initiative to Study Economic and Policy Implications of AgeTech
FPF and University of Arizona Eller College of Management Awarded Grant by Alfred P. Sloan Foundation to Address Privacy Implications, and Data Uses of Technologies Aimed at Aging At Home The Future of Privacy Forum (FPF) — a global non-profit focused on data protection, AI and emerging technologies–has been awarded a grant from the Alfred […]
Data Sharing for Research Tracker
Co-authored by Hannah Babinski, former FPF Intern In celebration of International Open Data Day, FPF is proud to launch the Data Sharing For Research Tracker, a growing list of organizations that make data available for researchers. It provides information about the company, the data, any access restrictions, and relevant links: One of the most difficult, […]
AI Forward: FPF’s Annual DC Privacy Forum Explores Intersection of Privacy and AI
The Future of Privacy Forum (FPF) hosted its inaugural DC Privacy Forum: AI Forward on Wednesday, June 5th. Industry experts, policymakers, civil society, and academics explored the intersection of data, privacy, and AI. In Washington, DC’s southwest Waterfront at the InterContinental, participants joined in person for a full-day program consisting of keynote panels, AI talks, […]
The Significance of Inclusion in Clinical Trials and Medical Research Databases
Our colleagues at the Israel Tech Policy Institute (ITPI) published a thoughtful blog on the significance of diversity and inclusion in clinical trials and health and medical research databases. They discuss the imperative of being represented in data, for one’s existence to be recognized and considered. When such data is the building block for a […]
Brain-Computer Interfaces: Privacy and Ethical Considerations for the Connected Mind
BCIs are computer-based systems that directly record, process, analyze, or modulate human brain activity in the form of neurodata that is then translated into an output command from human to machine. Neurodata is data generated by the nervous system, composed of the electrical activities between neurons or proxies of this activity. When neurodata is linked, or reasonably linkable, to an individual, it is personal neurodata.
FPF Health and AI & Ethics Policy Counsels Present a Scientific Position at ICML 2020 and at 2020 CCSQ World Usability Day
On November 12, 2020, FPF Policy Counsels Drs. Rachele Hendricks-Sturrup and Sara Jordan presented privacy-by-design alongside human-centered design concepts during the 2020 CCSQ World Usability Day virtual conference. This presentation followed Drs. Hendricks-Sturrup’s and Jordan’s July 2020 scientific position paper presented at the International Conference on Machine Learning (ICML) 2020, entitled “Patient- Reported Outcomes: A Privacy-Centric and Federated Approach […]
FPF Submits Comments Regarding Data Protection & COVID-19 Ahead of National Committee on Vital and Health Statistics Hearing
Yesterday, FPF submitted comments to the National Committee on Vital and Health Statistics (NCVHS) ahead of a Virtual Hearing of the Subcommittee on Privacy, Confidentiality, and Security on September 14, 2020. The hearing will explore considerations for data collection and use during a public health emergency, in light of the deployment of new technologies for […]
FPF & BrightHive Release Playbook to Create Responsible Contact Tracing Initiatives, Address Privacy & Ethics Concerns
A new playbook from the Future of Privacy Forum (FPF) and BrightHive, Responsible Data Use Playbook for Digital Contact Tracing, provides a series of considerations to assist stakeholders in setting up a digital contact tracing initiative to track and manage the spread of COVID-19, while addressing privacy concerns raised by these technologies in an ethical, […]
FPF, EFPIA, and CIPL Workshop Report Now Available: "Can GDPR Work for Health Scientific Research?"
On October 22, 2018, the Future of Privacy Forum (FPF), the European Federation of Pharmaceutical Industries and Associations (EFPIA), and the Centre for Information Policy Leadership (CIPL) hosted a workshop in Brussels, “Can GDPR Work for Health Scientific Research?” to discuss the processing of personal data for scientific research purposes under the European Union’s General Data Protection Regulation (GDPR).